Non-Exempt Studies with No Expiration
One of the major changes to the revised regulations for human research protection was removal of the requirement to conduct annual review (no expiration date) for some non-exempt studies. While annual review is not required for these studies, there are still continuing IRB responsibilities such as submission of modifications, reportable events, and closure of the study once it is complete. Once a year, principal investigators will receive an email reminder of these responsibilities for non-exempt studies approved by the MSU IRB with no expiration.
The Review Types and Process webpages for expedited and full board studies describe the requirements for modifications, reportable events, closure, and more. If you are unsure of whether you can close your study, the Closure webpage has detailed information and steps on how to submit a closure. Please contact the IRB office if you have any questions: irb@msu.edu.
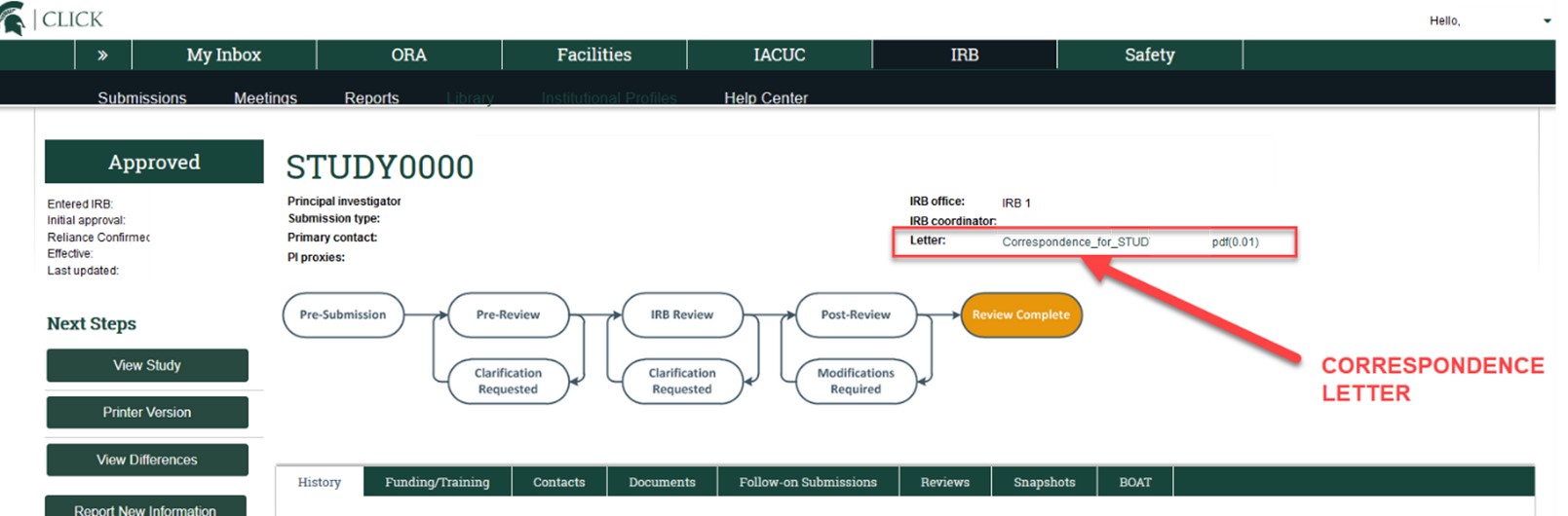
If you are unsure of whether your study is non-exempt with no expiration date, please find and open the study in MSU's IRB Online System, the Click(TM) Research Compliance System, and view the study’s most recent correspondence letter (either on the main study workspace or the most recent Modification’s workspace). A non-exempt study’s correspondence approval letters include the study’s review level (expedited or full board) and will indicate if the study does or does not have an expiration date. The correspondence approval letter is located on the top right of the study or modification’s workspace, labeled as “Letter” with a hyperlink.