Compliance Office
The HRPP Compliance office encompasses post approval monitoring activities, education, and non-IRB regulatory oversight (e.g. FDA requirements, protected health information, clinical research billing compliance).
Mission
The mission of the HRPP Compliance office is to assist in assuring compliance with all applicable federal, state and local regulations and university policies and procedures through quality independent and objective post approval monitoring, outreach, education, and oversight activities related to non-IRB regulatory requirements (e.g. FDA requirements, protected health information, clinical research billing compliance).
Functions
The HRPP Compliance office conducts pre and post approval reviews of applicable clinical research to provide oversight over regulatory areas that affect clinical research that are not addressed in the IRB requirements. For example, the HRPP would provide oversight of compliance with the FDA requirements for investigational new drugs and devices (21 CFR 312, 21 CFR 812), registration with clinicaltrials.gov (Public Law 110-85), appropriate storage of drugs and devices used in research, etc.
The Clinical Research Billing Compliance (CRBC) office is organized within the HRPP Compliance office and provides resources to the research community and others involved in patient care activities during research studies to ensure proper billing of health care services and items according to federal, state, and local regulations.
The HRPP Compliance office also conducts post approval monitoring activities, including site visits of human research projects.
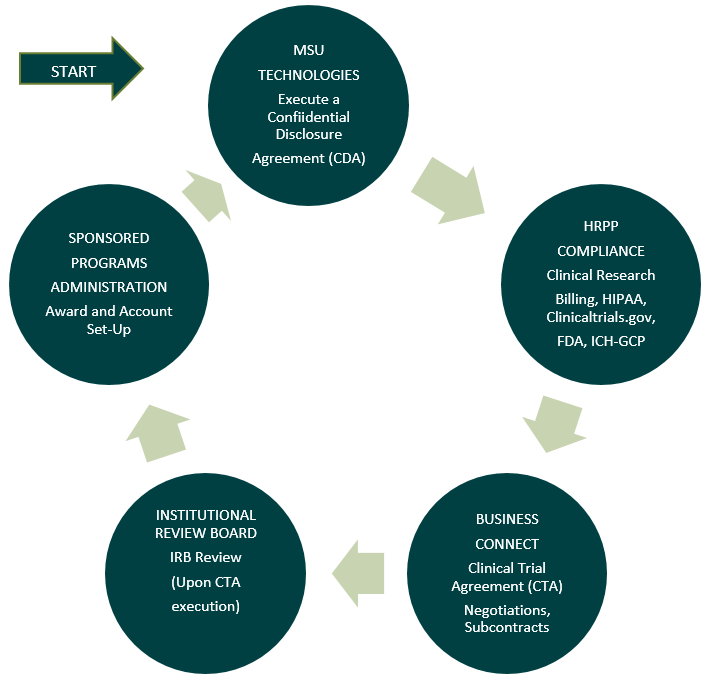
Flow diagram of MSU Administrative Offices involved with Industry Sponsored Research
Contacts
Please contact the HRPP Compliance Staff with any questions.

